Production
Established in 2012, L HOUSE is the largest cell culture vaccine plant in South Korea.
L HOUSE is capable of mass manufacturing
as the Korea's largest vaccine plant equipped with specialized technology.
as the Korea's largest vaccine plant equipped with specialized technology.
Capabilities
SK bioscience manufactures products under its technological expertise and cutting-edge facilities.
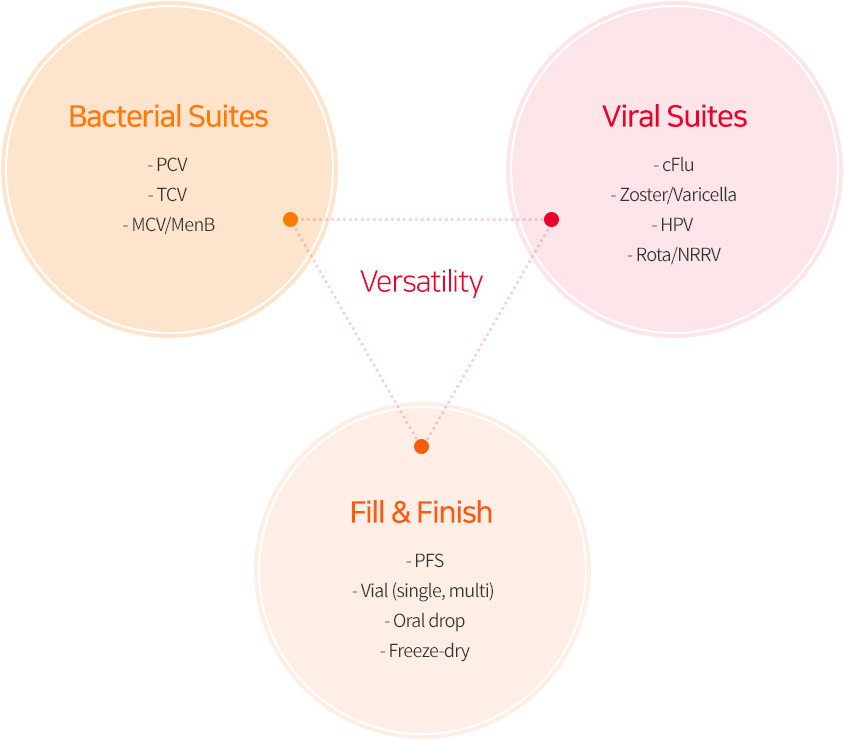
Versatility - Bacterial Suites, Viral Suites, Fill & Finish
- Bacterial Suites : PCV, TCV, MCV/MenB
- Viral Suites : cFlu, Zoster, Varicella, HPV, Rota, NRRV
- Fill & Finish : PFS, (single, multi), Oral drop, Freeze-dry
Pioneer in successful adaptation of ‘single-use system’ for vaccines on commercial scale
- 1. Flexibility in using single-use system
- Faster execution for process development & scale-up
- Cost effectiveness based on quick turnaround time, easy validation, without any cleaning process.
- 2. Diverse platforms
- Diverse vaccine production in one suite
- Flexible & rapid supply of clinical materials and commercial products
- 3. Future expansion
- Rapid expansion for capacity increase
‘Multi-modular system’ for diverse vaccine types
Fermentation
- E coli/yeast
- Polysaccharide
Protein conjugate
- DT/CRM197/TT
Cell culture
- Suspension
- Adherent
- Microcarrier
Recombination
- Commercial
- SKYCellflu
- SKYZoster
- SKYVaricella
- SKYCovione
- In-house R&D
- HPV
- Oral Rota
- Next PCV
- COVID 19
- Sarbecovirus
- Partnership R&D
- TCV
- NRRV
- COVID 19
- Sarbecovirus
