Press releases
-
 SK bioscience (CEO Jae-Yong Ahn) announced the promotion of new executives for 2022 on December 2nd...2021. 12. 2
SK bioscience (CEO Jae-Yong Ahn) announced the promotion of new executives for 2022 on December 2nd...2021. 12. 2 -
 SK bioscience will establish an industry-university partnership to preemptively respond to new vira...2021. 12. 2
SK bioscience will establish an industry-university partnership to preemptively respond to new vira...2021. 12. 2 -
 SK, IVI sign an agreement to launch the “Park MahnHoon Award” to honor individuals and organizations...2021. 11. 30
SK, IVI sign an agreement to launch the “Park MahnHoon Award” to honor individuals and organizations...2021. 11. 30 -
 The first submission of a protein-based COVID-19 vaccine candidate in KoreaHigh expectations of the ...2021. 11. 15
The first submission of a protein-based COVID-19 vaccine candidate in KoreaHigh expectations of the ...2021. 11. 15 -
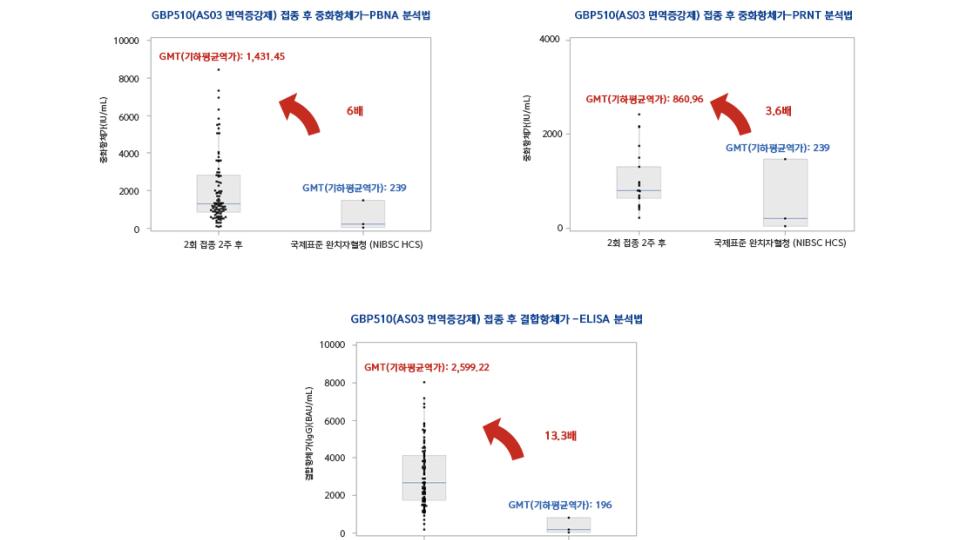 The final data of the phase I/II clinical trial confirms 3.6 to 6 folds of neutralizing antibody tit...2021. 11. 05
The final data of the phase I/II clinical trial confirms 3.6 to 6 folds of neutralizing antibody tit...2021. 11. 05 -
 SK bioscience will continue production of global biopharmaceutical company´s COVID-19 vaccines next...2021. 10. 06
SK bioscience will continue production of global biopharmaceutical company´s COVID-19 vaccines next...2021. 10. 06 -
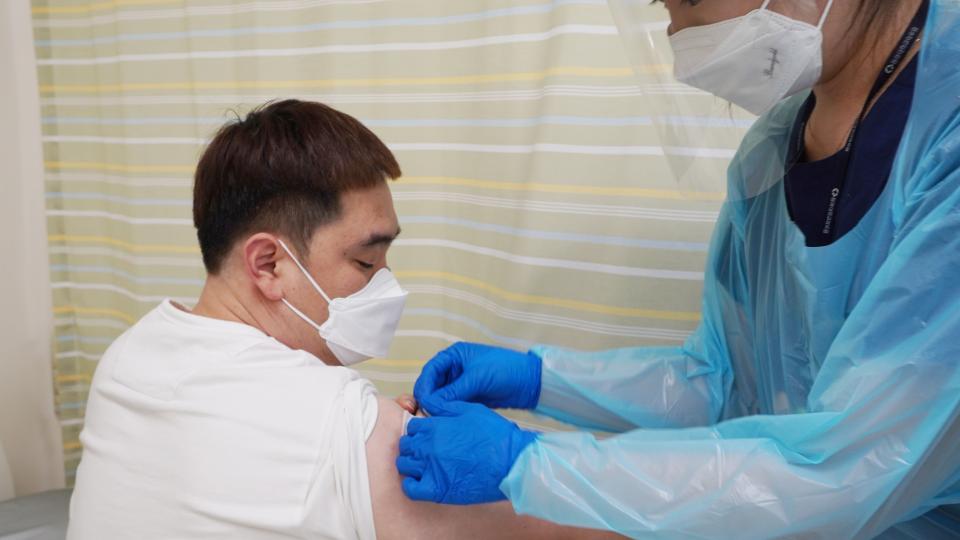 The Korean-developed COVID-19 vaccine GBP510, is administered to phase 3 clinical trial subjects f...2021. 08. 30
The Korean-developed COVID-19 vaccine GBP510, is administered to phase 3 clinical trial subjects f...2021. 08. 30 -
 - Phase I/II stage 1 confirms 5~8 folds of neutralizing antibody titer compared to human convalesce...2021. 08. 10
- Phase I/II stage 1 confirms 5~8 folds of neutralizing antibody titer compared to human convalesce...2021. 08. 10 -
 - GBP510, a COVID-19 vaccine candidate was submitted an IND for a Phase III clinical study in Korea ...2021. 06. 28
- GBP510, a COVID-19 vaccine candidate was submitted an IND for a Phase III clinical study in Korea ...2021. 06. 28 -
 ESG Committee will review major management strategies related to the environment, society, and gove...2021. 06. 24
ESG Committee will review major management strategies related to the environment, society, and gove...2021. 06. 24
